CO and CO2 – What’s the difference?
CO and CO2 – What’s the difference?
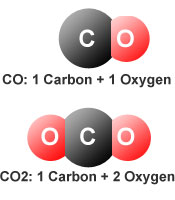
CO - carbon monoxide and CO2 - carbon dioxide are often confused. The names sound the same, they both are colorless and odorless gases, and at high concentrations, both can be deadly. The difference is that CO2 is a common, naturally occurring gas required for all plant and animal life. CO is not common. It is a byproduct of the oxygen-starved combustion of fuel.
The media often adds to the confusion. In the past, we heard stories of suicide by sticking a garden hose in a car's tailpipe and window, then gunning the motor till the CO (carbon monoxide) put the car's occupant to sleep. Today we are told our car’s tailpipe is a major source of the "deadly" greenhouse gas CO2. It's easy to see why they are confused.
It’s important that you understand the difference between CO and CO2:
About Carbon Monoxide
- CO does not occur naturally in the atmosphere
- CO is the result of oxygen-starved combustion in improperly ventilated fuel-burning appliances such as oil and gas furnaces, gas water heaters, gas ovens, gas or kerosene space heaters, fire places and wood stoves
- CO is generated by any gasoline engine that DOES NOT use a catalytic converter
- It is the most common type of fatal poisoning in the world
CO Recommended Levels
- OSHA limits long-term workplace exposure levels to 50ppm (parts per million)
- Symptoms of mild CO poisoning include headaches and dizziness at concentrations less than 100ppm
- Concentrations as low as 700ppm can be life-threatening
About Carbon Dioxide
- CO2 occurs naturally in the atmosphere, and is required for plant life
- CO2 is a natural byproduct of human and animal respiration, fermentation, chemical reactions, and combustion of fossil fuels and wood
- CO2 is generated by any gasoline engine that DOES use a catalytic converter
- CO2 poisoning is rare; however scuba divers have to watch out for it (the bends)
- Leaking compressed CO2 tanks in enclosed areas can be dangerous for occupants
CO2 Recommended Levels
- 400ppm is the current average CO2 level on the planet
- ASHRAE recommends a 1,000ppm limit for office buildings and classrooms
- OSHA limits long-term workplace exposure levels to 5,000ppm
- Drowsiness can occur at 10,000ppm – common in closed cars or auditoriums
- Symptoms of mild CO2 poisoning include headaches and dizziness at concentrations less than 30,000ppm (3%)
- At 80,000ppm (8%) CO2 can be life-threatening
Understanding PPM - parts per million
Parts-per-million is the way small numbers of molecules of gas in the air are typically measured, since their is much less than 1% of the molecules by volume. At 1% gas by volume, scientists will instead say 10,000ppm (10,000 / 1,000,000 = 1%). For example, It is easier write that the CO2 level in a room has risen from 400ppm to 859ppm than to write the CO2 level has risen from 0.04% to 0.0859%. However, both are correct.
How Monoxide and Dioxide Got their Names
You can thank the ancient Greeks for giving us their names for numerals:
• mono = 1
• di = 2
• tri = 3
• tetra = 4
• penta = 5
• hexa = 6
• hepta = 7
• octa = 8
• ennea = 9
• deca = 10
This is how we get English words like triangle (3 sides), the US Pentagon (a 5 sided-building) or decathlon (10 contests). So the first half of monoxide means 1 oxygen atom, and the first half of dioxide means 2 oxygen atoms.
For the second half of the word, we have oxide. Oxide is the name for a simple compound of oxygen with another element or group. For example, add oxygen to the element hydrogen and you get hydrogen dioxide (H20), or water. Other oxides you may have heard of are nitrous oxide (NO2 - laughing gas), or zinc oxide (ZnO - the active ingredient in sunscreen).
Fonte:
co2meter.com


